CDC Panel Delays Vote on Hepatitis B Vaccine
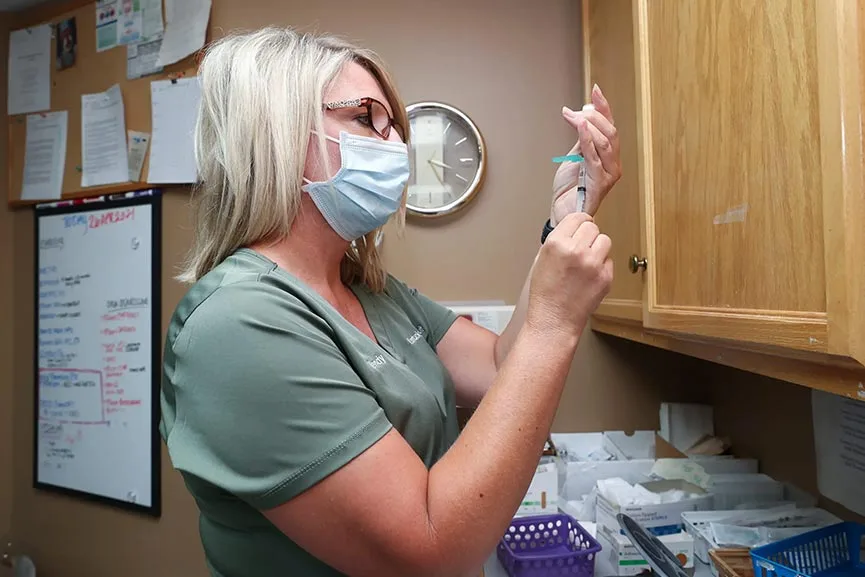
A vote on the hepatitis B vaccine schedule for newborns was unexpectedly delayed by the CDC’s Advisory Committee on Immunization Practices (ACIP) after meeting on Thursday.
According to attendees, technical glitches and confusion over voting language forced the panel to push the decision to Friday morning.
Confusion and Delays Disrupt CDC Panel Meeting
The main issue up for debate was whether to change the longstanding recommendation that all infants receive a hepatitis B vaccine within 24 hours of birth. Panel members faced logistical challenges, including multiple versions of voting questions and technical errors that left the final language unclear. Dr. Joseph Hibbeln, a panel member, expressed frustration, calling the process disorganized.
The proposal under review suggests shifting from universal vaccination to a selective approach, where only mothers testing positive for hepatitis B would ensure their newborns receive the vaccine. Critics argue this change could miss undetected cases and undo decades of progress. Since the CDC’s 1991 universal vaccine recommendation, hepatitis B infections in children have dropped by 99%.
Concerns Over Public Health Impact
Medical experts like Dr. Sean O’Leary of the American Academy of Pediatrics warn against changes, comparing the birth dose to a seatbelt: “No one expects to get in a car wreck, but we all wear seat belts.” Many fear altering the schedule could lead to preventable infections.
The panel’s new composition has also raised concerns. Critics, including Sen. Bill Cassidy , accuse the appointees of promoting vaccine skepticism. Some presentations during the meeting came from individuals described as anti-vaccine activists, breaking from the committee’s history of relying on CDC medical experts.
As the panel reconvenes, the medical community is watching closely, worried about potential changes to the vaccine that has been in place 34 years.
